Achieve Proactive Quality, Accelerate Time to Market, Improve Operational Efficiency and Effectiveness
For over 40 years, we’ve been a trusted partner to Life Sciences organizations, helping them to solve complex challanges while supporting their digital transformation journey. Our comprehensive solutions—spanning quality, manufacturing, cybersecurity, and more—are designed to enhance operational efficiency, reduce risk and empower Life Sciences companies to get safer products to market faster.
Transform Your Operations

Meet stringent regulatory requirements with integrated, automated solutions that maintain error-free audit trails, support inspections, and facilitate compliance, reducing the risk of non-compliance and delays to market.

Safeguard product quality and patient safety by utilizing built-in templates that align with ISO 14971 and ICH Q9. Proactively identify and mitigate risks, reducing errors and accelerating time to market.

Integrate your Quality Management System (QMS) and Manufacturing Execution System (MES) for error-free data flow and enhanced decision-making. This integration reduces time to market and boosts operational efficiency.

Address deviations in real-time, speeding up corrective actions and batch release processes. Move from weeks to hours, reducing errors and improving time to market without compromising compliance.

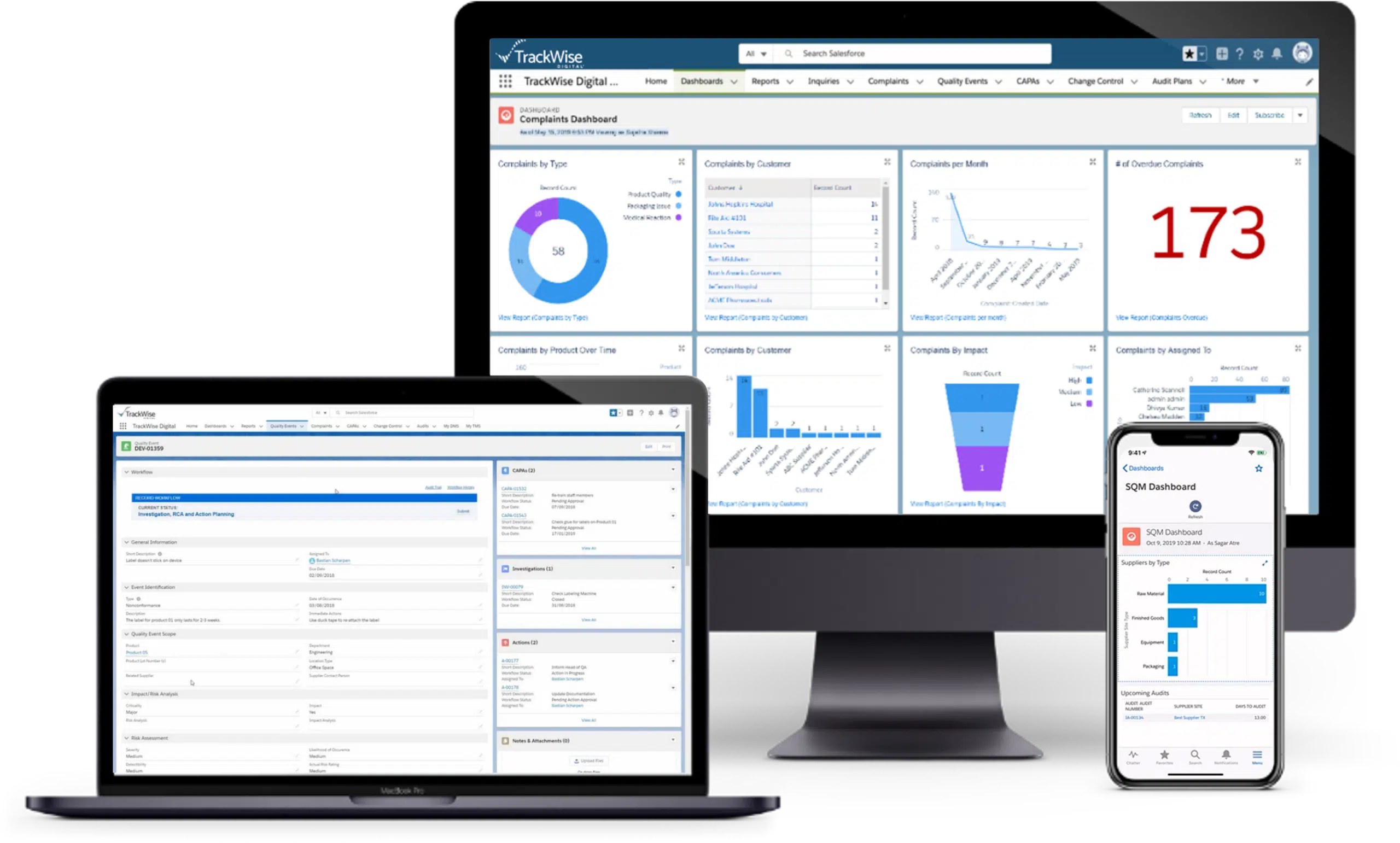
Monitor and gather valuable insights on product performance post-market, enabling effective management of customer complaints. Streamline regulatory reporting, reduce safety risks, and improve post-market processes for sustained compliance.
TRACKWISE
QUALITY SUITE
Quality is our foundation! Our Quality Management solutions help life sciences organisations bring products to market by leveraging the highest level of quality management processes and advanced AI-driven technologies, ensuring readiness for what’s next.
- TrackWise Digital
- TrackWise QMS
- TrackWise AI
- Product Quality Review (PQR)
- Quality Management Review (QMR)
TRACKWISE
MANUFACTURING SUITE
TrackWise Manufacturing offers advanced solutions to optimize production processes, ensure quality control, and drive operational excellence. Helping life sciences organizations modernize operations and achieve transformative results.
- Manufacturing Excellence Platform (MES)
- Experion Batch (DCS)
- Batch Historian
- Electronic Logbooks
- Electronic Batch Record

New Thought Leadership

SGS implemented TrackWise Digital to digitize and eliminate over 25,000 items, moving from a heavily paper-based and document-centric environment to connect over 17 sites and hundreds of partners on a state-of-the-art digital platform. Here’s why SGS chose TrackWise Digital.
- Intuitive user interface and easy-to-navigate dashboards
- Outstanding reputation and high use in the Life Science industry
- Comprehensive module with quality, complaints, documents, training, supplier quality and CAPA
- Robust audit management features that enable direct submission of audit replies, making it easier to conduct over 400 audits per year


Let’s talk about how TrackWise Quality and TrackWise Manufacturing can help
we have solutions for you

















































































 Proactive Quality >
Proactive Quality >
 Batch Operations for Improved Efficiency >
Batch Operations for Improved Efficiency >
 Efficient Product Quality Reviews >
Efficient Product Quality Reviews >
 Eliminating Paper Records >
Eliminating Paper Records >
 Proactive Quality Management Reviews >
Proactive Quality Management Reviews >
 AI-Augmented Quality Management >
AI-Augmented Quality Management >
 Data-Driven Insights for Batch Contextualization >
Data-Driven Insights for Batch Contextualization >
 Future-Ready Facilities >
Future-Ready Facilities >

